So, what is Hydroxyethylcellulose? Hydroxyethylcellulose is odorless, tasteless, and comes in white to light tan powders or granules.
COMMON NAME
Hydroxyethylcellulose or HEC is sometimes spelled with 2 words: hydroxyethyl cellulose. A check on EUR-Lex and CIR shows as one word, hydroxyethylcellulose.
TRADENAME / COMMERCIAL NAME
Natrosol™ (Ashland), HEC CF (Sumitomo Seika), Celopre® (Celotech), CELLOSIZE™ (Dow)
For the record, I am using Natrosol™ 250HHR.
INCI
Hydroxyethylcellulose
CAS
9004-62-0
FUNCTION
Thickening agent.
SOLUBILITY
Water soluble
PH
6.0 – 8.5
2% solution – 7.0
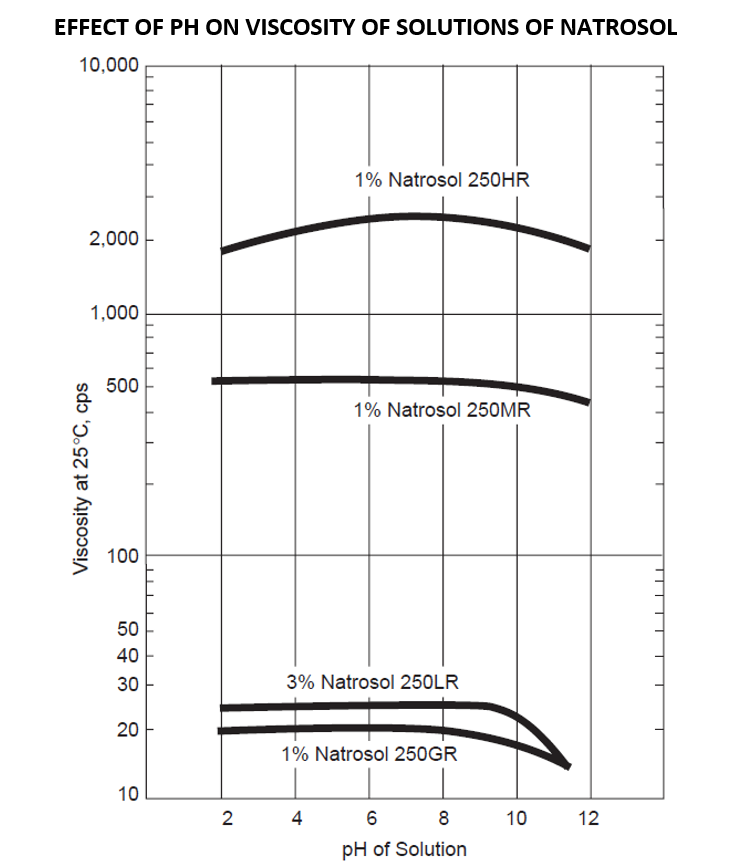
Covers a wide pH (3-10). It is less effected by pH change. However, the greatest viscosity stability is in the range of pH 6.5-8.0. Below pH 3, some viscosity may drop due to acid hydrolysis.
TEMPERATURE
Can be heated
Browning range.......... 205-210°C (401-410°F)
Softening range.......... 135-140°C (275-284°F)
CHARGE
Non-ionic
DESCRIPTION
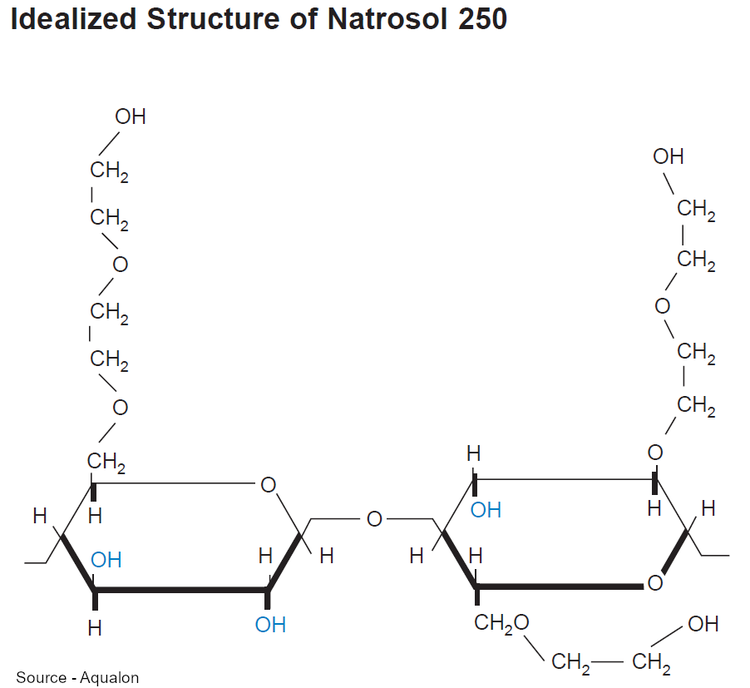
Hydroxyethylcellulose is a water-soluble polymer derived from cellulose. Cellulose is a natural sugar that is found in the cell wall of plants. In fact, cellulose is the most abundant organic sugar on earth. It is a semi-synthetic gelling agent, used as a thickening agent for aqueous cosmetic and personal care formulations. While cellulose is naturally derived, hydroxyethylcellulose is a hydroxyethyl ether of cellulose, synthetically made by reacting ethylene oxide with alkali-cellulose, a method known as ethoxylation. By reacting the cellulose with aqueous sodium hydroxide, cellulose ethers are made. Then, cellulose ethers react with an alkyl halide (such as methyl or ethyl chloride); and further reacted with propylene oxide or ethylene oxide to produce other types of cellulose.

One problem normally associated with water-soluble thickeners is the tendency of the particles to agglomerate or lump when first wetted with water. Hydroxyethylcellulose (Natrosol specifically) is available in several grades and types. Natrosol R-grade in particular, are surface-treated with a pH sensitive coating to delay hydration when it is added to water. This reduces lumping and agglomeration during processing. It is also the most efficient grade of non-ionic thickener available from the manufacturer (Ashland).

Since hydroxyethylcellulose is a biopolymer, therefore, many of its properties will depend on its molecular weight and the degree of substitution of hydroxyethyl or hydroxyl groups on the pyranose ring. Different viscosity of hydroxyethylcellulose can be blended to achieve an intermediate viscosity.
*Refers to Grade
B - Biostable
R - Retarded hydration
Pharm - Pharmaceutical (higher purity)
*Refers to the molecular weight and viscosity of the grade
HH - Very high
H - High
M - Medium
*Refers to the suitable applications for the grade
PC - Personal care
CS - Consumer product or Cosmetic grade
HC - Home care
*X and W refers to finer particle size
regular grind retained on 425 micrometer
X - 250 micrometer, fine
W - 177 micrometer, super fine
Hydroxyethylcellulose can be used in a variety of product applications. Hydroxyethylcellulose can be used as a thickener, protective colloid, binder, stabilizer and suspending agent in a variety of industrial applications, including pharmaceuticals, textiles, paper, adhesives, decorative and protective coatings, emulsion polymerization, ceramics and many other uses.
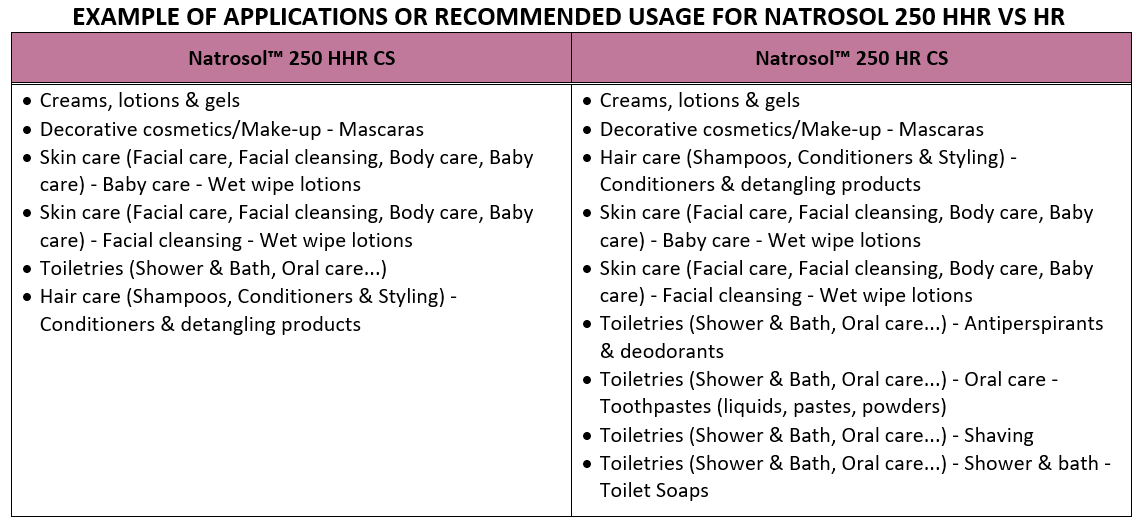
BENEFITS
- Hydroxyethylcellulose exhibits excellent salt tolerance. According to Ashland, sample formulations containing up to 20% salt have been successfully demonstrated.
- Can be used in a wide variety of products and industries.
- Broad surfactant compatibility.
- Prevents soil redeposition in cleaning applications.
- Nature-derived from cellulose, -54% natural origin content according to ISO16128-2:2017.
- Compatible with alcohol (up to 60% w/w).
- Imparts lubricious feel and slip in hair and skin care products.
- Covers a wide pH (3-10). It is less effected by pH change. However, the greatest viscosity stability is in the range of pH 6.5-8.0. Below pH 3, some viscosity may drop due to acid hydrolysis.
- More tolerant of the presence of anions and organic co-solvents
- It is soluble in hot or cold water and can also be dispersed in glycerin.
- It is compatible with most other gums but works especially well with sodium alginate.
- Natrosol does not display a “cloud” or “precipitation” point and does not precipitate from aqueous solution at elevated temperature.
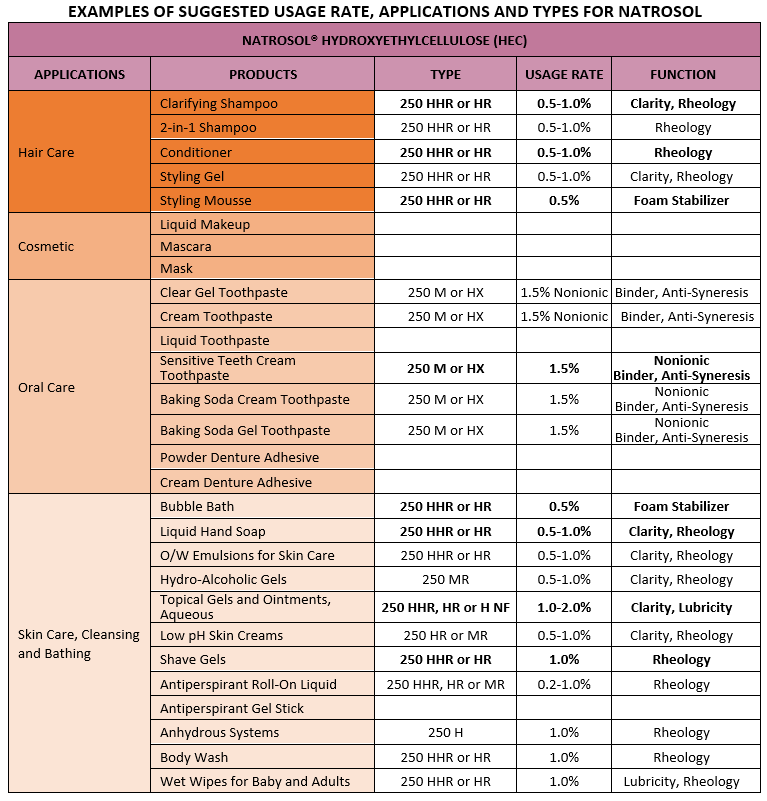
TYPICAL USAGE RATE
0.5 – 3%
USAGE GUIDELINES
Hydroxyethylcellulose is soluble in both cold and hot water, but under normal circumstances does not dissolve in most organic solvents. When the pH value is within the range of 2-12 (some say 3-10), the change in viscosity is small, but if beyond this range, the viscosity will decrease.
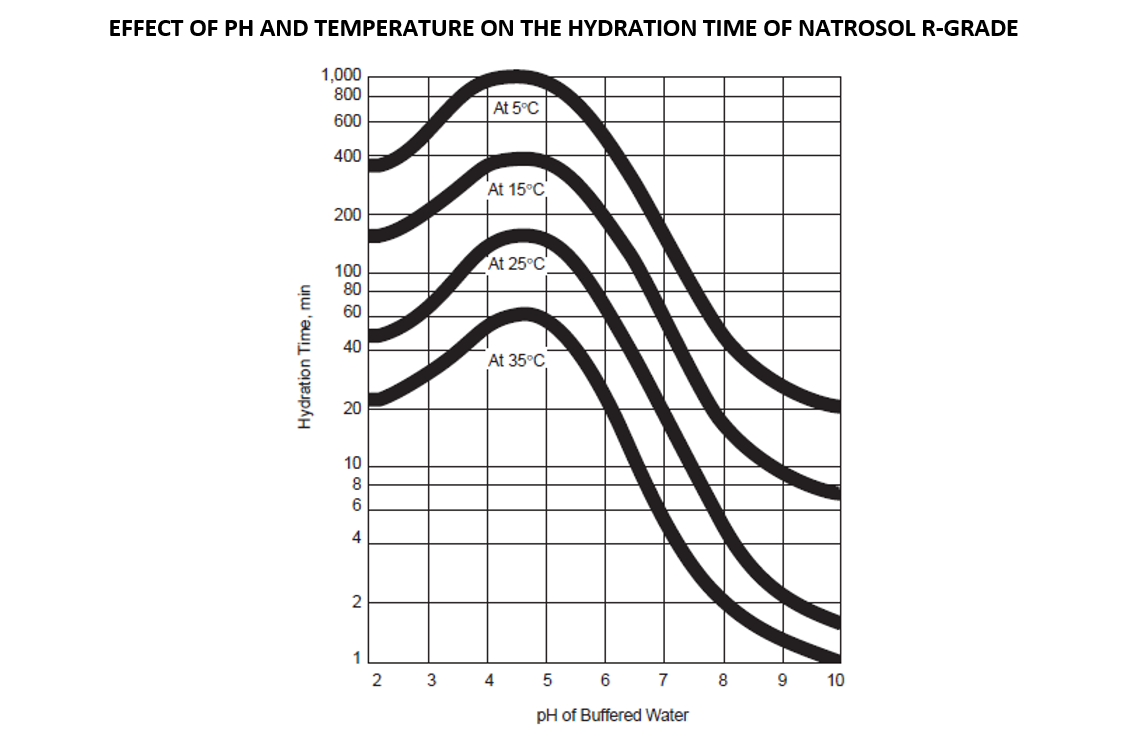
The hydration time can vary from a few minutes to an hour. The time is highly affected by two factors: pH and temperature of the water. Surface-treated hydroxyethylcellulose (such as the R-grade Natrosol) can be dispersed in cold water without agglomeration, but the dissolution rate is slower. With heat or adjusting the pH value to 8-10, it can be rapidly dissolved. Although a higher temperature and a higher pH decrease the hydration time, a temperature or pH that is too high can cause lumping. So, it is recommended that HEC be added to room temperature (RT) water with a neutral pH. Once hydrated, it can be heated and the pH can be adjusted as needed. It is ideal to add HEC at the beginning of the formulation to ensure that the polymer is completely hydrated before adding additional ingredients.
There are several ways on how to use surface-treated HEC.
Method 1 – Room Temperature, Neutral pH
At room temperature and neutral pH, HEC dissolves very slowly, and continuous stirring until the polymer is completely dissolved to prevent the settling of the particles is highly recommended. This method is the longest of the three. Using a magnetic stirrer or an overhead stirrer is recommended to free you from manual stirring.
While stirring, add HEC into the water, slow enough for the particles to separate in water without lump formation. Continue stirring until all swollen particles are dissolved to give a smooth solution. This process can take 40 minutes to an hour.
Method 2 - Room Temperature, Higher pH
While stirring, add HEC into the water, slow enough for the particles to separate in water without lump formation. Once powder is dispersed, adjust the pH to around 8.5 to trigger dissolution and viscosity build. Suitable neutralizers include NaOH (sodium hydroxide / lye), aminomethyl propanol (AMP) and triethanolamine (TEA). Continue stirring until the polymer is fully dissolved and a smooth solution texture is achieved. This usually take a few minutes. After HEC has fully dissolved, now you can adjust the pH back to your desired level by using citric acid or lactic acid solution.
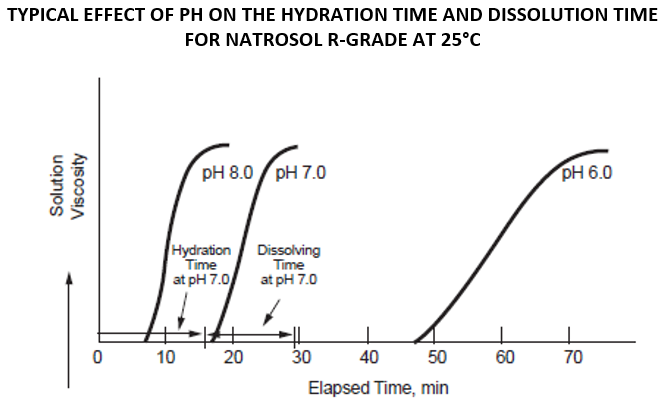
Viscosity will reduce under highly alkaline conditions (above pH 9.5) due to some oxidative degradation which may occur, which accelerated by heat and light.
Method 3 – Higher Temperature, Neutral pH
While stirring, add HEC into the RT water, slow enough for the particles to separate in water without lump formation. Once fully dispersed, heat to 80-90°C (176-194°F) while stirring until dissolved and a smooth solution texture is achieved. The viscosity changes with temperature, increasing when cooled, decreasing when warmed.
Other tips from Ashland
- If you are using non-surface treated grades, HEC can lump when added directly to water. Dispersing the polymer in a non-solvating liquid such as propylene glycol, propanediol, PEG, alcohols, at a polymer to liquid ratio of 1:5 before addition to aqueous phase is recommended.
- It is important to note that, as with any cellulose, HEC may decrease the antimicrobial activity of various preservatives. Care should be taken when finding an effective preservative concentration while ensuring that the concentration remains within the recommended limit. Some preservatives that have been found effective in laboratory tests are methyl-propylparaben combinations, sorbic acid, sodium benzoate, benzoic acid and benzalkonium chloride. Some of the preservatives used in Ashland sample formulas are Liquid Germall™ Plus, Optiphen™ BSB-W, Optiphen™ MIT Ultra and a few others.
Key Points to Remember
- Stir continuously while adding HEC into the water. Do not stop the mixer until the solution is clear and smooth.
- Add HEC into the water in a slow, smooth manner. Do not dump or shovel all in.
- Do not raise the pH before HEC is fully dispersed or wetted out.
- Add an effective preservative at the earliest convenient point in the process.
- Finally, always check the type and grade of hydroxyethylcellulose you are buying, as it will affect the process and outcome of your product.
STORAGE
Since the product is hygroscopic, stored under dry and clean conditions and away from heat. Ensure that the packaging selected is tightly closed to prevent the absorption of moisture. The water content of the packed product will/may increase if not stored properly.
Happy learning!
Reference
D.N.-S. Hon, Cellulose: Chemistry and Technology, Editor(s): K.H. Jürgen Buschow, Robert W. Cahn, Merton C. Flemings, Bernhard Ilschner, Edward J. Kramer, Subhash Mahajan, Patrick Veyssière, Encyclopedia of Materials: Science and Technology, Elsevier, 2001, Pages 1039-1045, ISBN 9780080431529, https://doi.org/10.1016/B0-08-043152-6/00192-3.
Kurup, T. R. R., L. S. C. Wan and Lai Wah Chan. “Interaction of preservatives with macromolecules. Part II. Cellulose derivatives.” Pharmaceutica Acta Helvetiae 70 (1995): 187-193.
www.celluloseether.com/physical-properties-of-hydroxyethyl-cellulose/
www.ashland.com
www.ulprospector.com
www.cosmetics.specialchem.com
#hydroxyethylcellulose #thickener


