I have heard cases where glassware has exploded in the microwave or been shattered into pieces by an overhead stirrer and before you start making snide comments, read this first (this writeup is for those who are new to formulating). If you are a seasoned formulator, you already know about this. As trivial as it may seem, it is important to know the right kind of glass even for your home cosmetic lab.
There are different types of glass available on the market, and choosing the right one depends on the usage and purpose. There are 2 types of glass commonly used in laboratories - Soda-Lime and Borosilicate glass. The chemical composition will differentiate each glass type and its uses.
- Expansion borosilicate glass – highly resistant to attack from acids, salt solutions, halogens and organic solvents. Only hydrofluoric acid, hot concentrated phosphoric acid and strong alkaline solutions cause significant corrosion.
- Neutral borosilicate glass (ASTM E-438 Type 1B) – excellent chemical resistance which makes it ideal for the storage or packaging of acidic, neutral and alkaline products and for injectable solutions.
- Soda-lime glass – less chemically resistant, moderately hydrolytically resistant, suitable for storing dry powders and for general sample storage applications.

First, let’s get to know borosilicate glass. Borosilicate glass is made by combining boron trioxide, silica sand, soda ash and alumina. They are produced in a wide range of compositions. If a significant amount of boric oxide is present in addition to silica, it is termed as borosilicate glass. Borosilicate glass is not only highly resistant to heat but also stands up to sudden temperature changes. Not that it doesn’t get hot when heated, but because of its lower thermal expansion when heated it does not expand like other non-borosilicate glasses, which are prone to shattering at high temperatures. Borosilicate glass was first made by German chemist and glass technologist Otto Schott in 1893, 22 years before Corning produced the Pyrex brand in 1915. Otto Schott is the founder of Schott AG, which sells the product under the name Duran. You can find many types of laboratory glassware made with borosilicate glass under the brands Pyrex, Bomex, Pallex, Schott, Iwaki and a few others. Borosilicate beakers are also available with a normal or thicker wall.
What are the advantages of borosilicate glass?
- Different composition of borosilicate glasses cover a wide range of different thermal expansions.
- Supports extreme temperatures ranging from -40°C to up to 500°C (depending on types).
- Thermal shock resistant up to 220°C. It can go directly from the freezer at a temperature of - 20°C into the oven at a temperature of 200°C.
- BPA free, hygienic material that doesn't retain stains or odors.
- Excellent chemical resistance, often used for scientific and medical laboratories.
- Higher electrical resistivity for certain industries (contains higher boron oxide).
- Scratch resistant.

Low-form (aka Griffin beaker) borosilicate glass is offered in slightly different compositions under different brands but mainly contain the same 13% of boron oxide

One of the main reasons people use glass in the lab is because of visibility. When doing lab work you want to see what is happening in the glassware whether for measurement purposes or to observe the reaction taking place. The second reason is that metal won’t react well with acids and certain other ingredients.
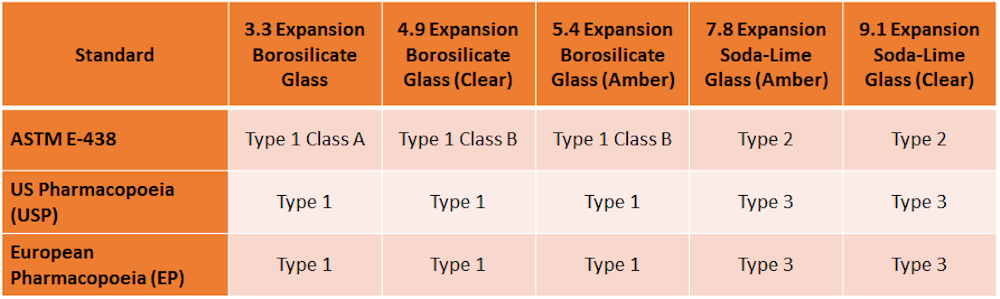
Different labware brands have their own reference numbers, but generally they refer to the same 3.3 Expansion Borosilicate Glass. For example, PYREX 7740 is the same as Schott 8330.

PYREX vs pYrex?
Older clear-glass Pyrex manufactured by Corning, Arc International's Pyrex products, and Pyrex laboratory glassware are made of borosilicate glass. However, beginning in the 1980s, production of clear Pyrex glass cookware manufactured by Corning was shifted to tempered soda-lime glass (commonly known as tempered glass). The main difference between soda-lime glass and borosilicate glass is their silicon dioxide and boron trioxide contents. Soda-lime glass has higher mechanical strength than borosilicate, therefore making it more resistant to physical damage when dropped. It is also cheaper to produce and more environmentally friendly. However, its thermal shock resistance is lower than that of borosilicate glass which will lead to potential breakage from heat stress. Borosilicate glass can withstand 2.5-3 times the temperature difference compared to soda lime silicate.
The European manufacturer of Pyrex, ARC International, still manufactures Pyrex with borosilicate which is differentiated by its different logo from American Pyrex.

Do you have one in your kitchen? If so, which one?
Whether it is soda lime or borosilicate glass, plasticware or stainless steel, ask yourself these questions before purchasing.
- Do you intend to use the container without heating?
- Does it involve low heat or high heat?
- Will you be using direct heating on a hotplate or microwave? Or will you be using a double boiler?
- What kind of chemicals will you be using?
- Is seeing the reaction to the content while processing important to you?
Then decide which one works for you or maybe you need all of them.
And then, there is also plastic labware (which will require another writeup)
So, regardless of whichever labware you choose, be it borosilicate glassware or plasticware, you need to remember that it has to be of high quality that can serve your purpose appropriately and therefore, it is crucial to find good quality glassware or plasticwares. Finally, like most materials, even borosilicate glass deteriorates over time, hence replacing old with new is advisable.
Get to know the equipment that you already have, so that you can use it appropriately based on its intended purpose, therefore minimizing any shattering or explosions.
Happy Learning!
Resources.
https://gizmodo.com/the-pyrex-glass-controversy-that-just-wont-die-1833040962
https://en.wikipedia.org/wiki/Pyrex
https://www.manufacturingchemist.com/news/article_page/Plastic_versus_glass_in_the_lab/81213
https://camblab.info/what-is-the-differences-between-glass-types-commonly-used-for-laboratory-glassware/
Eric Le Bourhis, Glass Mechanics and Technology, 2008, WILEY-VCH Verlag GmbH & Co. KGaA



Comments ()